ESMO 2022
ABSTRACT 818P: Phase 2 confirmatory study of cemiplimab (350mg IV Q3W) in patients with locally
advanced or metastatic cutaneous squamous cell carcinoma (CSCC): Study 1540 Group 6
Speaker: Brett G. Hughes
Background
While most patients diagnosed with CSCC are cured with local therapies, for the small percentage
developing advanced CSCC the disease is life threatening with dismal prognosis. In a phase 1 trial
and a pivotal phase 2 clinical trial, cemiplimab, an anti–programmed cell death receptor-1 [anti–PD-1],
was the first systemic therapy to demonstrate significant antitumor activity in patients with
advanced CSCC. Here, we report results from Group 6 of the pivotal phase 2 trial, providing additional
efficacy and safety data for cemiplimab monotherapy [350 mg every 3 weeks (Q3W)] up to 104 weeks,
in patients with advanced CSCC.
Methods
Patients with advanced CSCC (metastatic [nodal or distant] or locally advanced) were treated with
cemiplimab for up to 108 weeks. The primary endpoint was objective response rate (ORR; complete
response + partial response) per independent central review (ICR). Secondary endpoints included
duration of response (DOR), progression-free survival (PFS), and overall survival (OS) by central and
investigator review as well as safety and tolerability of cemiplimab.
Results
At data cut-off date of 25 October 2021, 167 patients were enrolled, of whom 165 received at least
one dose of cemiplimab and were followed-up for a median of 8.7 months (range: 0 to 19.5).
Their average age was 76 years (range, 40 to 68), and most had cancer of the head and neck (68%).
Five of these 167 patients had received prior systemic therapies for CSCC.
Per ICR, ORR was 45.1% (95% CI: 37.4% to 53.1%) with complete responses in 5.5% and partial
responses in 39.6%. The DOR was not reached (95% CI: 13.0 months to evaluable [NE]). Among treated
patients, median PFS was 14.7 months (95% CI: 10.4 to NE) and median OS was not reached (95% CI:
17.6 months to NE).
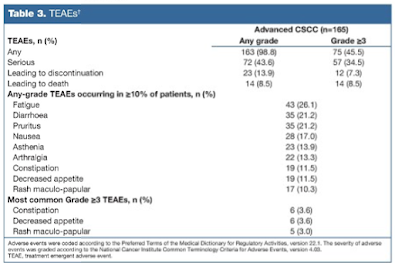
The most common treatment-emergent adverse events (TEAEs) of any grade were fatigue (26%),
diarrhea (21%), pruritus (21%), and nausea (17%). The most common grade ≥ 3 TEAEs were constipation and decreased appetite (each 3.6%), and maculo-papular rash (3%).
Conclusion
The Group 6 primary analysis demonstrates a safety and efficacy profile for cemiplimab that is
consistent with that of the earlier groups of Study 1540. Cemiplimab remains a standard-of-care option
in patients with advanced CSCC who are not candidates for curative surgery or radiation.
Funding: Regeneron Pharmaceuticals and Sanofi

Comments
Post a Comment